About osteonecrosis of the femoral head (ONFH)
ONFH is a refractory disease that causes femoral head collapse, pain, limitation of hip motion and gait disturbance.
Patient characteristics
<Age at presentation>
30 years of age
<Incidence of
bilateral cases>
50%
<Risk factors>
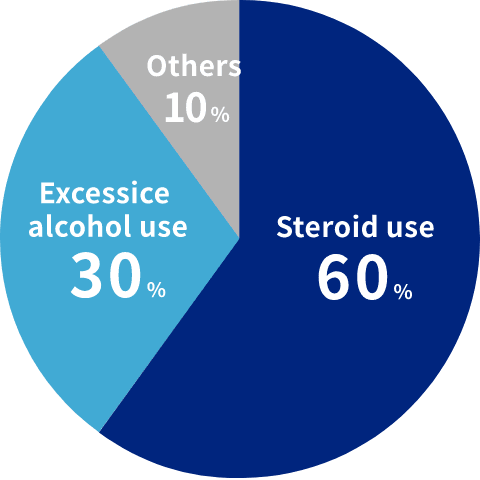
The main cause of the disease is the use of steroids.
About
ONFH is a destructive disease of the hip joint caused by a critical decrease in the vascular supply to the femoral head.
Disadvantages of artificial joints
Disadvantages of artificial joints

Joint preserving regenerative therapy
has been the ultimate therapeutic goal for ONFH.
Attention needs to be paid to a possible rapid increase
in steroid-associated ONFH after the COVID-19 pandemic
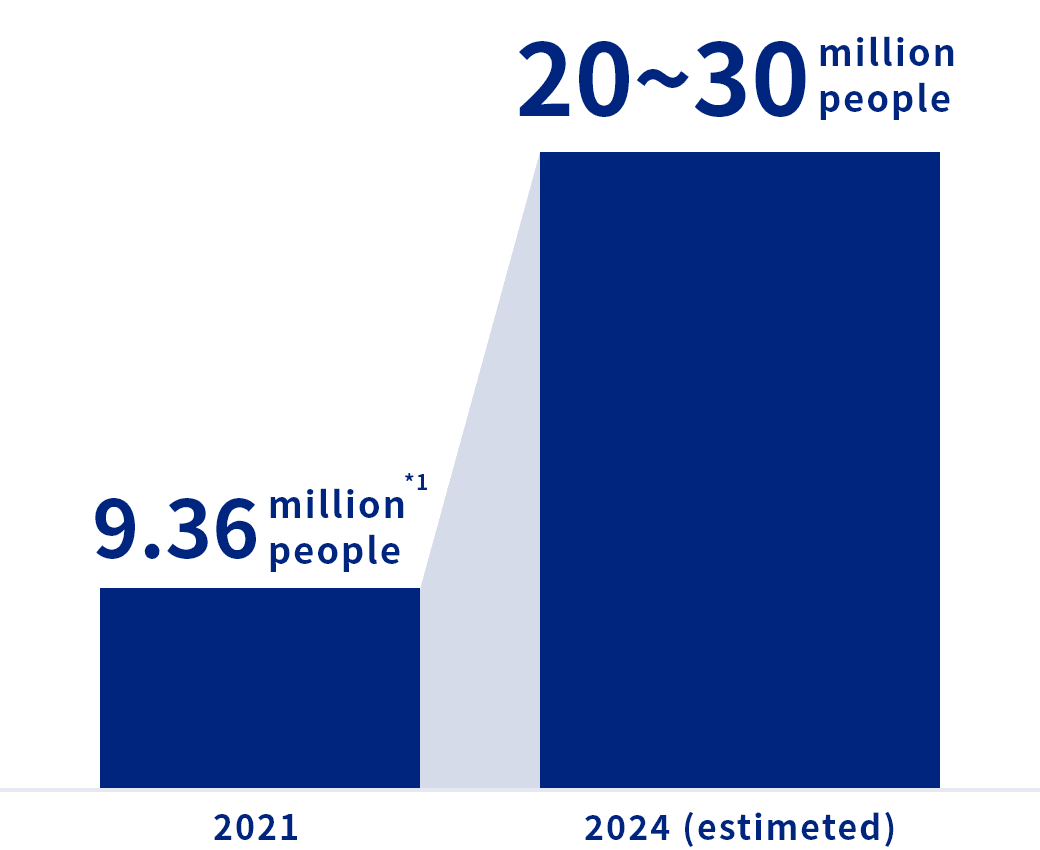
※1 References: Ikeuchi K, Hasegawa Y, Seki T, Takegami Y, Amano T, Ishiguro N. Epidemiology of nontraumatic osteonecrosis of the femoral head in Japan. Mod. Rheumatol. 25(2), 278-281 (2015).
Pointed by the articles※2
- 01. Steroid use is a common cause of ONFH
- 02. Steroid therapy for COVID-19 pheumonia
- 03. During the SARS outbreak,
24% of patients developed ONFH.
※2 References:Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. Tang C, Wang Y, Lv H, Guan Z, Gu J. Caution against corticosteroid-based COVID-19 treatment. Lancet 2020; 395:1759-1760. Sodhi N, Acuna A, Etcheson J, et al. Management of osteonecrosis of the femoral head. Bone Joint J 2020;
We offer the following values:
Regenerative therapy for ONFH
-

Regenerative medical products
based on research resultsResearch and development of high-spec adipose tissue-derived stem cells, growth factors, exosomes, and cellular pads. By ensuring the fixation of growth factors and cells at the time of injection, it is possible to maintain their effects for a longer duration.
-

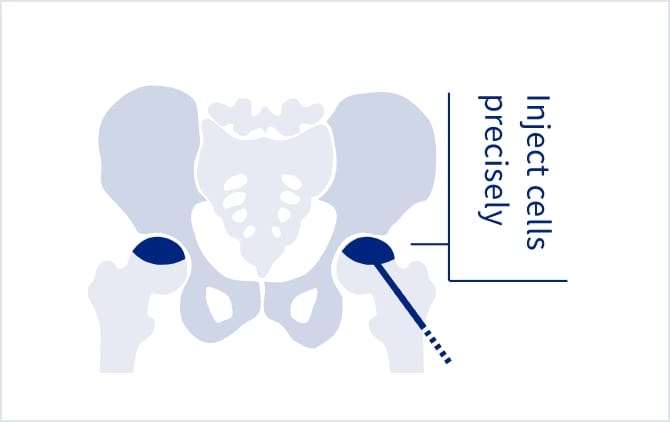
Original
surgical devicesUse of novel surgical devices and the development of novel devices for precise cell injection (patent pending). Since the skin incision made during the treatment is only 1 cm long, day treatment is possible. Moreover, we are developing products using environmentally friendly materials.
-

Regenerative medicine
consultingWe support wider use of “regenerative medicine” using cells and growth factors. We provide complete support for clinical management, branding, and technical guidance to both insured and uninsured patients.
The regenerative technologies developed for ONFH will be applied to the treatment of osteoarthritis of the knee, bone fractures, and osteoporosis.
Comparison
| Parameter※1 | Conventional treatments | Treatment offered by Cell Factor※2 |
|---|---|---|
| Surgical treatment |
Total hip replacement | Regenerative therapy using cells and growth factors |
| Hospitalization | 2~3 weeks | 2~3 days |
| Skin incision |
10cm | 1cm |
| Operation time |
1 hour~ | 5 minutes |
| Medical costs | 10 million JPY※3 | 2 million JPY~ |
| Difficulty of surgery |
Differences by surgical experience | Specialized device allows even resident surgeons to perform surgery accurately |
※1 For the patients with ONFH.
※2 There are cases that cannot be treated if the symptoms progress.
※3 Total medical costs including revision surgeries.
First, it is applied to treatment based on the Regenerative Medicine Act. Subsequently, we aim to promote the use of the system through insurance.
The Envisioned Future
Cell Factor will not only enhance the well-being of patients with “osteonecrosis of the femoral head” but also of those with various other diseases and maladies.
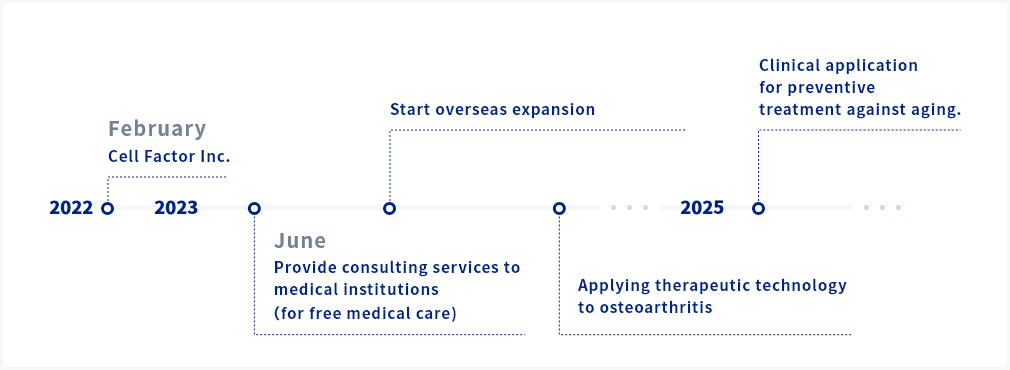
Application has already started for developing the preventive treatment against aging. It is difficult to secure developers for research in the treatment of intractable and rare diseases since this field may not in line with corporate interests. Cell Factor will overcome this challenge and continue to safely provide the regenerative medicine needed by the patients and society.